TRI’s KL2 Mentored Research Career Development Scholars Program announced nine new scholars for 2022-2023, its largest-ever class.
These promising early-career researchers receive two years of funded support and mentored translational research training. The program selects scholars through a competitive application process and provides 75% salary support and up to $25,000 a year for research, tuition, travel and education.
Additional scholars were selected this year thanks to funding support from the College of Medicine, Winthrop P. Rockefeller Cancer Institute, Arkansas Children’s Research Institute and Central Arkansas Veterans Healthcare System.
The scholars, their project titles and primary mentors are:
Jennifer Andersen, Ph.D.

Jennifer Andersen, Ph.D., assistant professor, Northwest Regional Campus, Office of Community Health and Research
“Feasibility and Acceptability of a Remote Glucose Monitoring Program for Pregnant Marshallese Women whose Pregnancies are Complicated by Diabetes”
Primary Mentor: Hari Eswaran, Ph.D.
Timothy “Cody” Ashby, Ph.D., M.S.

Timothy “Cody” Ashby, Ph.D., M.S., assistant professor, College of Medicine Department of Biomedical Informatics
“Determining Multiple Myeloma Risk and Heterogeneity at a Single-Cell Resolution”
Primary Mentor: Fenghuang Zhan, M.D., Ph.D.
Nishank Jain, M.D.
Nishank Jain, M.D., assistant professor, College of Medicine Department of Internal Medicine, Division of Nephrolog
“Platelet, Inflammation and Thrombosis in Chronic Kidney Disease”
Primary Mentor: John Arthur, M.D., Ph.D.
Akilah Jefferson-Shah, M.D., M.Sc.

Akilah Jefferson-Shah, M.D., M.Sc., assistant professor, College of Medicine Department of Pediatrics, Division of Allergy and Immunology.
“Delineating Individual and Population-level Factors that Contribute to Disparate Pediatric Asthma Outcomes and to Develop Predictive Models for Identifying Children at Risk for Poor Outcomes”
Primary Mentor: Tamara Perry, M.D.
Nakita Lovelady, Ph.D., MPH

Nakita Lovelady, Ph.D., MPH, assistant professor, Fay W. Boozman College of Public Health, Department of Health Behavior and Health Education.
“A Feasibility Study for the Implementation of a Hospital-based Violence Intervention Program in the Rural South”
Primary Mentor: Nickolas Zaller, Ph.D.
Sayem Miah, Ph.D.

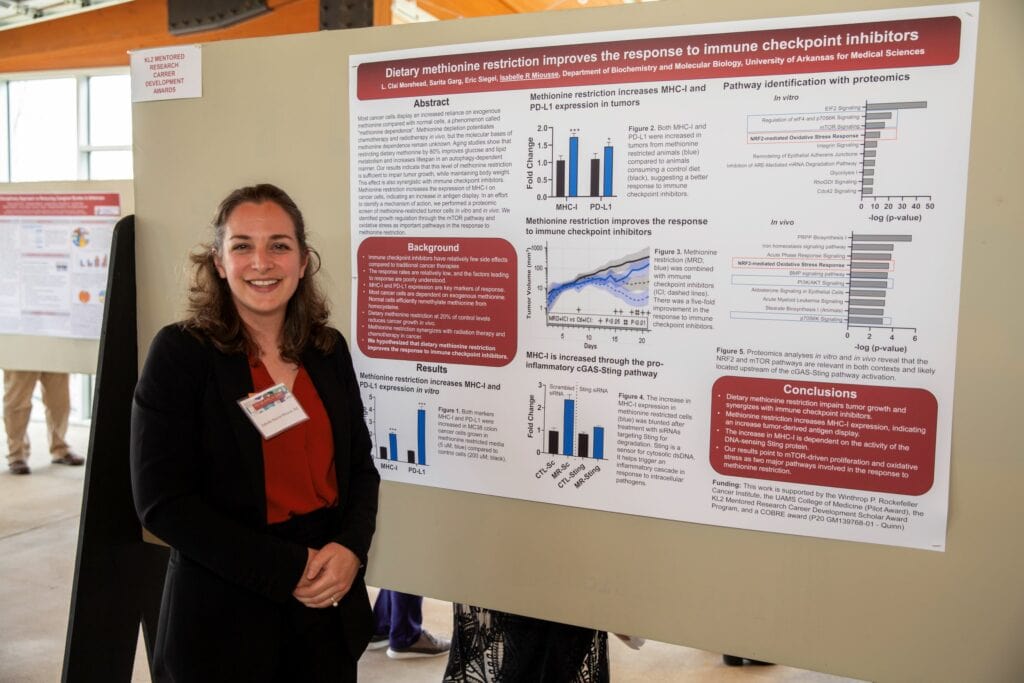
Sayem Miah, Ph.D., assistant professor, College of Medicine Department of Biochemistry and Molecular Biology
“Targeting BRK with PROTAC to Halt Metastatic Triple Negative Breast Cancer”
Primary Mentor: Alan Tackett, Ph.D.
Deepa Raghavan, M.D.

Deepa Raghavan, M.D., assistant professor, College of Medicine Department of Internal Medicine, Division of Pulmonary and Critical Care; medical director, VA Medical ICU
“Implementation of COPD Clinical Practice Guidelines with Incorporation of Telehealth”
Primary Mentor: JoAnn Kirchner, M.D.
Jennifer Rumpel, M.D.

Jennifer Rumpel, M.D., assistant professor, College of Medicine Department of Pediatrics, Neonatology Section
“Advancing Care of Neonates with Acute Kidney Injury Utilizing the Children’s Hospitals Neonatal Consortium Database”
Primary Mentor: Laura James, M.D.
Amy Sato, Ph.D.

Amy Sato, Ph.D., assistant professor, College of Medicine Department of Physiology and Cell Biology
“Identification of Cardioprotective Signatures Induced by Targeting MuRF1 and Vitamin D Signaling in Glucocorticoid-Associated Cardiac Disease”
Primary Mentor: Marjan Boerma, Ph.D.