Harvard Medical School’s Mark Bauer, M.D., a national leader in research design and facilitating models of implementation, will be the visiting lecturer April 11, 12:30 – 1:30 p.m., COPH, 8/240. His presentation is “Applying Implementation Science Concepts in Real-World Studies.”
Front
March TRIbune Features ‘Lean’ Endeavor on Behalf of Researchers
The March TRIbune newsletter features TRI’s embrace of Lean training to achieve more efficiency in the services it provides to researchers. The TRI Leadership Council participated in a day-long workshop on the key concepts of Lean. Also in this issue, read how TRI-support helped Kristie Hadden, Ph.D., land her first NIH grant for a health literacy/diabetes education study; TRI Director Laura James, M.D., writes about TRI’s plans for the year and recent progress; Pedro Delgado, M.D., is our TRI & Me feature; and see recent publication citations by TRI-supported researchers.
It’s all in The TRIbune.
Profiles Training and Troubleshooting, April 26
UAMS Profiles is offering hands-on training and troubleshooting April 26 for those who want to enhance their profile page as well as those with technical issues to resolve. In addition, the training is open to any staff who may be assisting faculty with their profiles.
TRI’s Shawna Owens Receives Bonny Hope Wallace Award
Feb. 16, 2016 | The UAMS Translational Research Institute’s (TRI) Shawna Owens, C.R.A., C.C.R.P.,
C.R.S., has received the 2015 Bonny Hope Wallace Award for her outstanding work with research participants.
Owens is TRI’s research coordinator unit manager and has been at UAMS for 20 years, starting in pediatrics, then the Winthrop P. Rockefeller Cancer Institute prior to joining TRI.
The Bonny Hope Wallace Award goes to a UAMS certified research specialist selected by their peers. Wallace worked in research at UAMS for more than 30 years before her death in 2004. The award in her name was presented at the conclusion of the Feb. 12 Certified Research Specialist (CRS) Awards Ceremony, sponsored by the UAMS Office of Research Compliance.
Owens’ nomination letter states that “Shawna is one of the most dedicated research coordinators at UAMS. She works tirelessly to provide the best possible experience for her participants and upholds the highest standards for research integrity in her interactions with both subjects and investigators. Her colleagues find her knowledgeable and helpful in all things related to human subjects research and rely on her wisdom and experience for guidance on a regular basis. Shawna is an expert in campus research policies and processes and is eager to help others navigate the system.”
Wallace was an instructor in surgery and laboratory director for surgical research at the Department of Surgery at UAMS as well as clinical coordinator of research at the Arkansas Children’s Hospital (ACH) Burn Unit. She was instrumental in pioneering ACH’s Burn Unit. Her efforts were focused on cutting-edge research to promote women’s health. She is remembered for her respectful treatment of research participants and her commitment to research integrity.
Owens said she was among those lucky enough to know “Miss Bonny” personally. “She was one of those amazing people that truly loved people and was completely dedicated to her work. It’s an honor to win an award named for her.”
When presenting the award to Owens, Sandy Annis, director of the Clinical Trials Office at the Cancer Institute, acknowledged the work of UAMS’ 104 certified research specialists. “Over this past year, research became personal for me as my mother was diagnosed with breast cancer and was treated on a research protocol,” she said. “I saw everything we all do in a completely different way. I want to thank all of you for what you are a part of and for the dedication and sacrifice that all of you make so that everyone will gain advancements in medical care and so that all our families and friends will get the care they deserve.”
Annis noted that constantly changing regulations and increasing complexity of clinical research challenge those in the field. Of Owens, she said, “She is highly respected among her peers and has continued to grow and show dedication to the reason we are all a part of this complex chaos.”
Certifications Awarded for UAMS Research Staff
Feb. 16, 2016 | Fourteen University of Arkansas for Medical Sciences (UAMS) employees have earned Certified Research Specialist (CRS) certificates. The recipients were announced at a Feb. 12 ceremony.
The certification program, administered by the UAMS Office of Research Compliance, ensures an understanding of, and respect for, the principles of research integrity and the protection of those who participate in research. Although the certification is not required by all departments, UAMS research employees routinely complete the 26 hours of coursework and the comprehensive CRS proficiency exam.
The recipients are:
- Kathryn Allen, Cancer Clinical Trials Office
- Michael Bailey, Translational Research Institute
- Scott Crump, COM Research and Evaluation Division
- Barbara Curtis, Central Arkansas Veterans Health System
- Leanna Delhey, COM Pediatrics Neurology Research
- Jessica Gann, Myeloma Institute
- Audrie Johnston, Arkansas Children’s Hospital Quality Improvement
- Jami Jones, Cancer Clinical Trials Office
- Jennifer McCluskey, Office of Research Compliance
- Leila Montague, Regional Programs and Grants Administration
- Shemeka Randle, Arkansas Children’s Hospital
- Jenika Sanchez, Otolaryngology Clinical Support
- Robert Smith, Myeloma Institute
- Ty Stacey, Myeloma Institute
In addition, 90 UAMS employees were acknowledged for maintaining their CRS certification, which requires that they remain current on Collaborative Institutional Training Initiative (CITI) Human Subject Protection training and complete six hours of continuing education each calendar year.
Nationally Acclaimed Implementation Science Experts to Lecture at UAMS April 1 and 11
Enola Proctor, Ph.D., a national leader in implementation science at Washington University in St. Louis, will present “Implementation Science: New Frontiers” on April 1, 12:30 – 1:30 p.m., College of Public Health (COPH), 8/240.
Sponsored by TRI, Proctor’s presentation is one of two Implementation Science Visiting Lecturers scheduled for April.
Proctor has a broad range of implementation research expertise and leads Washington University’s Clinical and Translational Science Award (CTSA) core in dissemination and implementation research. She is also director of the dissemination and implementation initiative at the university’s Institute for Public Health. She is associate dean for faculty and the Frank J. Bruno Professor of Social Work Research in the George Warren Brown School of Social Work.
Harvard Medical School’s Mark Bauer, M.D., a national leader in research design and facilitating models of implementation, will be the visiting lecturer April 11, 12:30 – 1:30 p.m., COPH, 8/240. His presentation is “Applying Implementation Science Concepts in Real-World Studies.”
A professor of psychiatry at Harvard, Bauer is also associate director of the Center for Healthcare Organization and Implementation Research (CHOIR), at the Department of Veterans Affairs (VA) Boston Healthcare System.
UAMS’ Efforts Getting Experimental Drug for Patient ‘Unheard of’

Jan. 27, 2016 | It was Christmas night and Yogesh Jethava, M.D., was worried.
Working the holiday at UAMS Medical Center, he had just diagnosed a leukemia patient’s rare, life-threatening liver disease. The only known treatment was a drug awaiting U.S. Food & Drug Administration approval and not available at most medical centers.
What he thought would be a straightforward emergency-use request to the pharmaceutical company that developed the drug, turned into a near impossible hurdle when he was told that UAMS would have to go through the complex process of opening a clinical trial to receive the drug.
That’s when an extraordinary effort by dedicated UAMS employees from multiple offices began to unfold.
Opening a clinical trial typically takes months, and UAMS’ research support offices were closed for the long holiday weekend. Although Jethava had alerted the appropriate people, he wasn’t expecting what happened next.
While most people were busy sampling leftover pie Christmas night, UAMS’ Sandy Annis and Jennifer Roberts were at their home computers catching up on work.
Annis, who leads the Clinical Trials Office for the Winthrop P. Rockefeller Cancer Institute, saw Jethava’s request in an email from Roberts, director of the Research Pharmacy.
“Jennifer and I have worked together for so long she knew who to contact,” Annis said. “Luckily Jennifer included several of the appropriate people on campus and had already talked to the company.”
The following day Annis completed work on about 30 documents for the drug company. She also drafted an emergency-use informed consent document for the patient to sign. Any hope of getting the clinical trial approved quickly would also require involvement and approval from other UAMS officials, including Suzanne Alstadt, director of the Office of Research and Sponsored Programs, Dori Wong-Scoggins, senior contracts attorney, and Jennifer Holland, director of the Institutional Review Board office.
“In my 12 years in this office, this is the first time we’ve ever attempted to get something like this accomplished on a holiday or a weekend,” Annis said. “Amazingly everybody was on email and they were responsive. The drug company was, too.”
They worked by email and text, often using their smart phones. Wong-Scoggins was traveling in California that Saturday after Christmas. She used a smartphone to negotiate and edit the agreement with the drug company.
“I was a passenger in the car going up to Napa with my family,” Wong-Scoggins said. “Editing on a smartphone app is doable, but it’s harder. I was getting car sick.”
Holland, who was driving home from Tennessee the same day, counted more than 60 emails and text messages. When her UAMS email inbox reached capacity on her phone, she switched to her gmail account.
“Talk to text was a lifesaver,” Holland said. “I processed the IRB acknowledgement letter at a gas station parking lot somewhere between Nashville and Memphis.”
The biggest challenge, Holland said, was working through the drug company’s requirements.
“I’ve worked on several of these types of emergency-use situations in the past 15 years, and this is the first time we’ve ever been asked to fully execute a clinical trial agreement,” she said. Despite the obstacles, the clinical trial agreement was approved and the drug, Defibrotide, was at UAMS three days later.
Jethava said he was amazed at the extraordinary efforts of so many research staff. “This is the most remarkable thing that can happen,” Jethava said. “It is unheard of.”
Annis attributed the accomplishment to the group’s many years of working together, dedication to UAMS, and trust in each other.
“I don’t think this would have worked if even one person wasn’t a player,” she said. “It took everybody.”
She said there was no consideration of waiting until the following Monday to work on the request.
“It never crossed our minds,” she said. “We knew this patient was in a bad situation and there wasn’t any other alternative medically. We would expect the same if it had been our own family.”
NCATS Invites Two TRI-Supported Collaborative Proposals
When the call went out in 2015 for innovative collaboration ideas involving the Clinical and Translational Science Award (CTSA) consortium, UAMS researchers joined their CTSA colleagues to offer six proposals.
Two of those proposals are moving to the next phase; full UO1 applications were recently invited by the NIH National Center for Advancing Translational Sciences (NCATS). The two are led locally by UAMS Translational Research Institute (TRI)-supported researchers Mary Aitken, M.D., M.P.H., and Mathias Brochhausen, Ph.D. “I am very proud of all the researchers who submitted proposals, and I am excited by the two selected to go forward,” said Laura James, M.D., TRI director. “This new NCATS initiative has provided a great opportunity to showcase our expertise in translational research and our ability to work effectively in a collaborative national network.”
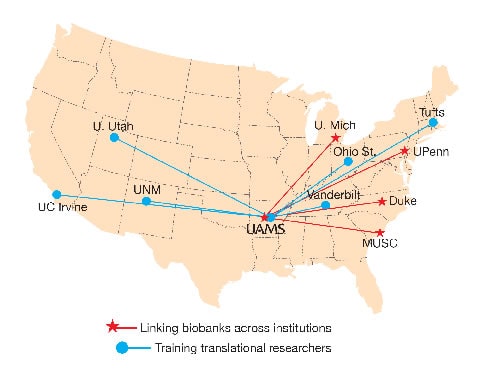
Linking Biobank Data
Brochhausen, principal investigator for the UAMS site, views his U01 collaboration with four other CTSA institutions as an opportunity to achieve the nationally elusive dream of making biobank data from multiple institutions available to researchers across the United States.

“We think now we have the right group of people to actually address the issue,” said Brochhausen, an assistant professor in the Department of Biomedical Informatics.
Other collaborating sites are Duke University, Medical University of South Carolina, University of Michigan, and University of Pennsylvania.
Biobanks include collections of biospecimens and data from electronic health records . Access to multiple sources of biobank data is expected to be a strong driver of biomarker discovery, hypothesis generation and new therapeutics. The biggest hurdle has been the lack of standard terminology among biobanks, Brochhausen said. To address the challenge, the collaborative developed an “integrative semantic framework,” with a common language for biobank data. Its proposal also integrates local informed consent procedures for donor specimens.
“For translational research, that is really significant because our proposal will allow researchers to query multiple databases from multiple sites,” Brochhausen said. “With informed consent as part of this program, we’ll reduce delays by weeding out query results that researchers can’t use.”
Educating Translational Researchers
Aitken is the UAMS site principal investigator collaborating with researchers at six other institutions to develop and improve upon educational curricula and tools supporting the training of translational scientists. The collaborating sites are the University of Utah, Ohio State University, Tufts University, University of California, Irvine, University of New Mexico, and Vanderbilt University.
“Our preliminary proposal was well received,” said Aitken, co-director of TRI’s KL2 Mentored Research Career Development Scholar Award program. “The full proposal will allow more detail about the courses to be offered across the consortium.”

The proposal, which targets KL2, TL1 and other trainees, calls for further developing the best education programs at each institution. It includes TRI-supported implementation science, regulatory science and community engagement as areas of training that UAMS could offer to other institutions.
“The idea is to provide courses lasting up to fivedays that trainees could travel to,” said Aitken, a professor in the Department of Pediatrics. “Telemedicine and online options are also likely.”
The collaborative’s proposal also calls for the development of preconference short courses that could be offered in conjunction with the annual Association for Clinical and Translational Sciences (ACTS) meeting each April.
Aitken is working closely with other UAMS research and education leaders on the project, including Geoffrey Curran, Ph.D., Jay Gandy, Ph.D., Laura James, M.D., Robert E. McGehee, Ph.D., Nancy Rusch, Ph.D., and Kate Stewart, M.D., M.P.H.
If approved, each research program will receive up to $500,000 a year for five years.
January 2016 TRIbune
The January TRIbune newsletter features two TRI-supported collaborations with multiple institutions across the country. The collaborations led at UAMS by Mary Aitken, M.D., M.P.H., and Mathias Brochhausen, Ph.D., were invited to submit full applications in February by the NIH National Center for Advancing Translational Sciences (NCATS).
Also in this issue, TRI Director Laura James, M.D., writes about ARresearch.org, a TRI-sponsored participant recruitment website. A key feature of the website is a registry for people to sign up as potential research participants. You’ll also read about a BioVentures startup with TRI connections, PinPoint Testing LLC; new KL2 Scholars Bryce Marquis, Ph.D., and Shona Ray-Griffith, M.D.; a TRI perspective from Jean McSweeney, Ph.D., R.N.; and recent publications of TRI-supported researchers. It’s all in The TRIbune.
Download Newsletter | Newsletter Archive

UAMS Profiles Training Dates Set
Four trainings are scheduled in February and March for faculty and post-doctoral researchers interested in learning how to use UAMS Profiles, UAMS’ new web-based researcher networking tool.
The 1-hour hands-on trainings will be conducted Feb. 23, noon – 1 p.m. and 4 – 5 p.m.; March 2, 4 – 5 p.m.; and March 8, noon – 1 p.m.
All trainings will be held in EDII 8/105 A/B. Register in Training Tracker. Sign up now – each training is limited to 30 participants. Contact: Nia Indelicato, nlindelicato@uams.edu.


