When the call went out in 2015 for innovative collaboration ideas involving the Clinical and Translational Science Award (CTSA) consortium, UAMS researchers joined their CTSA colleagues to offer six proposals.
Two of those proposals are moving to the next phase; full UO1 applications were recently invited by the NIH National Center for Advancing Translational Sciences (NCATS). The two are led locally by UAMS Translational Research Institute (TRI)-supported researchers Mary Aitken, M.D., M.P.H., and Mathias Brochhausen, Ph.D. “I am very proud of all the researchers who submitted proposals, and I am excited by the two selected to go forward,” said Laura James, M.D., TRI director. “This new NCATS initiative has provided a great opportunity to showcase our expertise in translational research and our ability to work effectively in a collaborative national network.”
Linking Biobank Data
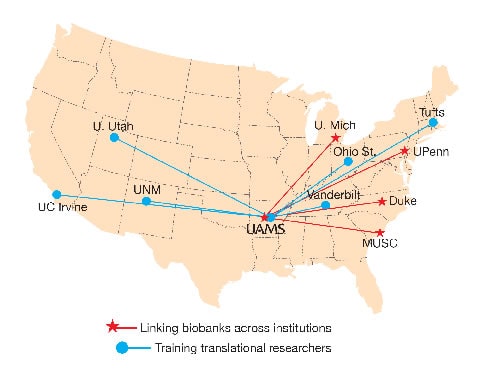
Brochhausen, principal investigator for the UAMS site, views his U01 collaboration with four other CTSA institutions as an opportunity to achieve the nationally elusive dream of making biobank data from multiple institutions available to researchers across the United States.

“We think now we have the right group of people to actually address the issue,” said Brochhausen, an assistant professor in the Department of Biomedical Informatics.
Other collaborating sites are Duke University, Medical University of South Carolina, University of Michigan, and University of Pennsylvania.
Biobanks include collections of biospecimens and data from electronic health records . Access to multiple sources of biobank data is expected to be a strong driver of biomarker discovery, hypothesis generation and new therapeutics. The biggest hurdle has been the lack of standard terminology among biobanks, Brochhausen said. To address the challenge, the collaborative developed an “integrative semantic framework,” with a common language for biobank data. Its proposal also integrates local informed consent procedures for donor specimens.
“For translational research, that is really significant because our proposal will allow researchers to query multiple databases from multiple sites,” Brochhausen said. “With informed consent as part of this program, we’ll reduce delays by weeding out query results that researchers can’t use.”
Educating Translational Researchers
Aitken is the UAMS site principal investigator collaborating with researchers at six other institutions to develop and improve upon educational curricula and tools supporting the training of translational scientists. The collaborating sites are the University of Utah, Ohio State University, Tufts University, University of California, Irvine, University of New Mexico, and Vanderbilt University.
“Our preliminary proposal was well received,” said Aitken, co-director of TRI’s KL2 Mentored Research Career Development Scholar Award program. “The full proposal will allow more detail about the courses to be offered across the consortium.”

The proposal, which targets KL2, TL1 and other trainees, calls for further developing the best education programs at each institution. It includes TRI-supported implementation science, regulatory science and community engagement as areas of training that UAMS could offer to other institutions.
“The idea is to provide courses lasting up to fivedays that trainees could travel to,” said Aitken, a professor in the Department of Pediatrics. “Telemedicine and online options are also likely.”
The collaborative’s proposal also calls for the development of preconference short courses that could be offered in conjunction with the annual Association for Clinical and Translational Sciences (ACTS) meeting each April.
Aitken is working closely with other UAMS research and education leaders on the project, including Geoffrey Curran, Ph.D., Jay Gandy, Ph.D., Laura James, M.D., Robert E. McGehee, Ph.D., Nancy Rusch, Ph.D., and Kate Stewart, M.D., M.P.H.
If approved, each research program will receive up to $500,000 a year for five years.