The trial start-up process at an academic medical center like UAMS can be a complex process. The Clinical Trials Innovation Unit (CTIU) can help investigators navigate the steps. To support an efficient start-up process, the CTIU offers investigators a well organized team-based approach. This approach serves as a systematic means to coordinate the work of multiple experts simultaneously working on the same goal. This team driven approach relies heavily on common work sharing tools, teamwork, communication, and accountability.
The CTIU staff have expertise in feasibility analyses, protocol development management, Medicare coverage analyses, budget development and negotiations, regulatory management, study coordinator and nursing support. The CTIU also works collaboratively with other groups on campus, such as attorneys for research contracts and institutional compliance offices as well as coordinates protocol submissions to central and local institutional review boards (IRBs).
Accessing Feasibility
Prior to starting an industry-sponsored study, the investigator and/or their department should evaluate the study on the basis of the research interests of the individual investigator and the perceived availability of research participants. Another part of this evaluation should include the administrative feasibility of conducting each trial.
The (CTIU) recommends using the Feasibility and Study Planning Guide to assess these questions and all other applicable sections for study feasibility.
Trial sponsors typically request their own feasibility questionnaires after a confidentially agreement is executed. These questionnaires help sponsors decide on site selections and will include questions about the study team’s experience, potential participants, facilities, and study start-up timelines. A CTIU Project Manager is available to help answer feasibility questionnaires from trial sponsors.
Pre-Study Visits (PSV)
After the CDA is signed and a feasible assessment is started a pre-study visit (PSV) also know as a qualification visit or site selection visit may be required by the sponsor. A sponsor may use a pre-study visit to determine if a) the site is adequate, b) the investigator and research team are motivated, experienced, and capable of conducting the clinical trial, and c) if access to the patient population is adequate. The principal investigator is required to attend at least a portion of this visit. The sponsor may request a tour of research facilities and present the protocol for questions from the study team during this visit.
Site Initiation Visits (SIV)
Most sponsors will require a site initiation visit (SIV). The purpose of this visit is to prepare a research site and get them ready to conduct the study. This occurs prior to participant recruitment. The principal investigator must attend this visit and it is strongly recommended that all of the research team also attend. During this visit the research team will receive training on the study protocol design. Responsibilities regarding study procedures, regulations and documentation of study activities are discussed to ensure agreement and understanding. The sponsor’s representative may answer any of the study team’s questions at this time.
CTIU’s Clinical Research Team offers support to help investigators schedule and prepare for these visits and will assist them throughout each visit.
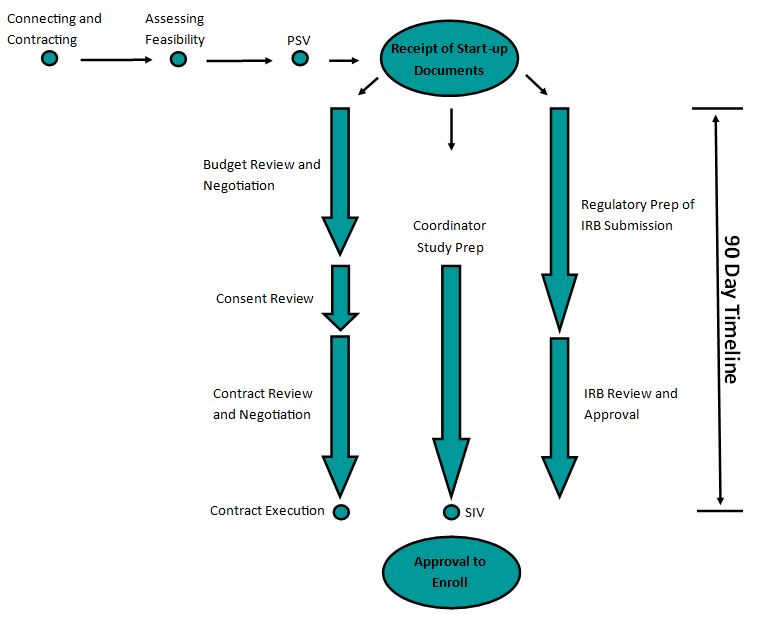
The Trial Start-up Process Overview
The CTIU overall timeline goal is 90 days from the time all start-up documents are received to an executed contract. The budget will be negotiated and agreed upon before the consent will be edited. Once the consent and budget are agreed upon, contract negotiation will begin. Submission to the IRB can be in parallel to the contract negotiation. The CTIU can provide investigators access to the team members who expertly navigate the steps.