[youtube id=”https://www.youtube.com/watch?v=2zMYsUQvQg4″ width=”300″ height=”200″ autoplay=”no” api_params=”” class=””][/youtube]
Uncategorized
UAMS’ Efforts Getting Experimental Drug for Patient ‘Unheard of’

Jan. 27, 2016 | It was Christmas night and Yogesh Jethava, M.D., was worried.
Working the holiday at UAMS Medical Center, he had just diagnosed a leukemia patient’s rare, life-threatening liver disease. The only known treatment was a drug awaiting U.S. Food & Drug Administration approval and not available at most medical centers.
What he thought would be a straightforward emergency-use request to the pharmaceutical company that developed the drug, turned into a near impossible hurdle when he was told that UAMS would have to go through the complex process of opening a clinical trial to receive the drug.
That’s when an extraordinary effort by dedicated UAMS employees from multiple offices began to unfold.
Opening a clinical trial typically takes months, and UAMS’ research support offices were closed for the long holiday weekend. Although Jethava had alerted the appropriate people, he wasn’t expecting what happened next.
While most people were busy sampling leftover pie Christmas night, UAMS’ Sandy Annis and Jennifer Roberts were at their home computers catching up on work.
Annis, who leads the Clinical Trials Office for the Winthrop P. Rockefeller Cancer Institute, saw Jethava’s request in an email from Roberts, director of the Research Pharmacy.
“Jennifer and I have worked together for so long she knew who to contact,” Annis said. “Luckily Jennifer included several of the appropriate people on campus and had already talked to the company.”
The following day Annis completed work on about 30 documents for the drug company. She also drafted an emergency-use informed consent document for the patient to sign. Any hope of getting the clinical trial approved quickly would also require involvement and approval from other UAMS officials, including Suzanne Alstadt, director of the Office of Research and Sponsored Programs, Dori Wong-Scoggins, senior contracts attorney, and Jennifer Holland, director of the Institutional Review Board office.
“In my 12 years in this office, this is the first time we’ve ever attempted to get something like this accomplished on a holiday or a weekend,” Annis said. “Amazingly everybody was on email and they were responsive. The drug company was, too.”
They worked by email and text, often using their smart phones. Wong-Scoggins was traveling in California that Saturday after Christmas. She used a smartphone to negotiate and edit the agreement with the drug company.
“I was a passenger in the car going up to Napa with my family,” Wong-Scoggins said. “Editing on a smartphone app is doable, but it’s harder. I was getting car sick.”
Holland, who was driving home from Tennessee the same day, counted more than 60 emails and text messages. When her UAMS email inbox reached capacity on her phone, she switched to her gmail account.
“Talk to text was a lifesaver,” Holland said. “I processed the IRB acknowledgement letter at a gas station parking lot somewhere between Nashville and Memphis.”
The biggest challenge, Holland said, was working through the drug company’s requirements.
“I’ve worked on several of these types of emergency-use situations in the past 15 years, and this is the first time we’ve ever been asked to fully execute a clinical trial agreement,” she said. Despite the obstacles, the clinical trial agreement was approved and the drug, Defibrotide, was at UAMS three days later.
Jethava said he was amazed at the extraordinary efforts of so many research staff. “This is the most remarkable thing that can happen,” Jethava said. “It is unheard of.”
Annis attributed the accomplishment to the group’s many years of working together, dedication to UAMS, and trust in each other.
“I don’t think this would have worked if even one person wasn’t a player,” she said. “It took everybody.”
She said there was no consideration of waiting until the following Monday to work on the request.
“It never crossed our minds,” she said. “We knew this patient was in a bad situation and there wasn’t any other alternative medically. We would expect the same if it had been our own family.”
NCATS Invites Two TRI-Supported Collaborative Proposals
When the call went out in 2015 for innovative collaboration ideas involving the Clinical and Translational Science Award (CTSA) consortium, UAMS researchers joined their CTSA colleagues to offer six proposals.
Two of those proposals are moving to the next phase; full UO1 applications were recently invited by the NIH National Center for Advancing Translational Sciences (NCATS). The two are led locally by UAMS Translational Research Institute (TRI)-supported researchers Mary Aitken, M.D., M.P.H., and Mathias Brochhausen, Ph.D. “I am very proud of all the researchers who submitted proposals, and I am excited by the two selected to go forward,” said Laura James, M.D., TRI director. “This new NCATS initiative has provided a great opportunity to showcase our expertise in translational research and our ability to work effectively in a collaborative national network.”
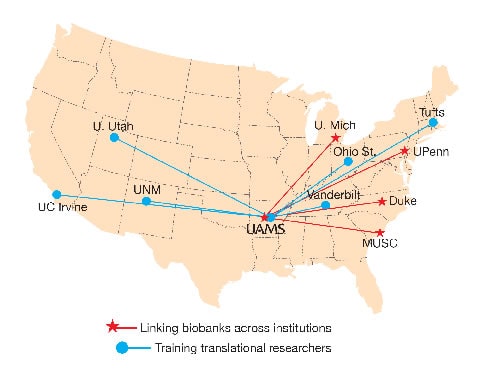
Linking Biobank Data
Brochhausen, principal investigator for the UAMS site, views his U01 collaboration with four other CTSA institutions as an opportunity to achieve the nationally elusive dream of making biobank data from multiple institutions available to researchers across the United States.

“We think now we have the right group of people to actually address the issue,” said Brochhausen, an assistant professor in the Department of Biomedical Informatics.
Other collaborating sites are Duke University, Medical University of South Carolina, University of Michigan, and University of Pennsylvania.
Biobanks include collections of biospecimens and data from electronic health records . Access to multiple sources of biobank data is expected to be a strong driver of biomarker discovery, hypothesis generation and new therapeutics. The biggest hurdle has been the lack of standard terminology among biobanks, Brochhausen said. To address the challenge, the collaborative developed an “integrative semantic framework,” with a common language for biobank data. Its proposal also integrates local informed consent procedures for donor specimens.
“For translational research, that is really significant because our proposal will allow researchers to query multiple databases from multiple sites,” Brochhausen said. “With informed consent as part of this program, we’ll reduce delays by weeding out query results that researchers can’t use.”
Educating Translational Researchers
Aitken is the UAMS site principal investigator collaborating with researchers at six other institutions to develop and improve upon educational curricula and tools supporting the training of translational scientists. The collaborating sites are the University of Utah, Ohio State University, Tufts University, University of California, Irvine, University of New Mexico, and Vanderbilt University.
“Our preliminary proposal was well received,” said Aitken, co-director of TRI’s KL2 Mentored Research Career Development Scholar Award program. “The full proposal will allow more detail about the courses to be offered across the consortium.”

The proposal, which targets KL2, TL1 and other trainees, calls for further developing the best education programs at each institution. It includes TRI-supported implementation science, regulatory science and community engagement as areas of training that UAMS could offer to other institutions.
“The idea is to provide courses lasting up to fivedays that trainees could travel to,” said Aitken, a professor in the Department of Pediatrics. “Telemedicine and online options are also likely.”
The collaborative’s proposal also calls for the development of preconference short courses that could be offered in conjunction with the annual Association for Clinical and Translational Sciences (ACTS) meeting each April.
Aitken is working closely with other UAMS research and education leaders on the project, including Geoffrey Curran, Ph.D., Jay Gandy, Ph.D., Laura James, M.D., Robert E. McGehee, Ph.D., Nancy Rusch, Ph.D., and Kate Stewart, M.D., M.P.H.
If approved, each research program will receive up to $500,000 a year for five years.
January 2016 TRIbune
The January TRIbune newsletter features two TRI-supported collaborations with multiple institutions across the country. The collaborations led at UAMS by Mary Aitken, M.D., M.P.H., and Mathias Brochhausen, Ph.D., were invited to submit full applications in February by the NIH National Center for Advancing Translational Sciences (NCATS).
Also in this issue, TRI Director Laura James, M.D., writes about ARresearch.org, a TRI-sponsored participant recruitment website. A key feature of the website is a registry for people to sign up as potential research participants. You’ll also read about a BioVentures startup with TRI connections, PinPoint Testing LLC; new KL2 Scholars Bryce Marquis, Ph.D., and Shona Ray-Griffith, M.D.; a TRI perspective from Jean McSweeney, Ph.D., R.N.; and recent publications of TRI-supported researchers. It’s all in The TRIbune.
Download Newsletter | Newsletter Archive

UAMS Profiles Training Dates Set
Four trainings are scheduled in February and March for faculty and post-doctoral researchers interested in learning how to use UAMS Profiles, UAMS’ new web-based researcher networking tool.
The 1-hour hands-on trainings will be conducted Feb. 23, noon – 1 p.m. and 4 – 5 p.m.; March 2, 4 – 5 p.m.; and March 8, noon – 1 p.m.
All trainings will be held in EDII 8/105 A/B. Register in Training Tracker. Sign up now – each training is limited to 30 participants. Contact: Nia Indelicato, nlindelicato@uams.edu.
New Drug Just Part of International Effort to Combat Castleman Disease
Probably few people would say Carl Guenther’s diagnosis was lucky, although he says so, and he has agreement from the world’s foremost expert on Castleman disease, Frits van Rhee, M.D., Ph.D., at the University of Arkansas for Medical Sciences (UAMS).
Guenther, a Wilmington, Ohio, father of two, was diagnosed with Castleman disease and given two years to live just months after his wife died of ovarian cancer. The lucky part? His doctor, who had recently completed medical training, remembered the disease from textbooks and correctly diagnosed it – no easy feat for a rare disease with no formal diagnostic criteria, van Rhee said.
“The average oncology doctor may see one Castleman disease patient in their lifetime, so you cannot possibly expect them to be an expert on a really rare disease,” van Rhee said. “In my opinion, people will die of this disease because they will not get the correct diagnosis, the diagnosis will be delayed, or they will receive inappropriate treatment.”
Patients with Castleman disease overproduce lymphocytes, leading to enlarged lymph nodes, and they have night sweats and fever. They may also have liver failure, renal failure, accumulation of fluid in the chest and abdomen, respiratory failure and death. The tumors that can result from the disease are usually benign, but Castleman can progress to malignant lymphoma.
“Although it’s a lymph node disorder, sometimes lymph nodes can look like Castleman disease due to other disorders, particularly diseases like lupus and autoimmune disorders,” van Rhee said. “So just looking at the microscope and saying ‘This looks like Castleman disease’ is not sufficient.”
Having the right diagnosis helped Guenther find van Rhee, who 10 years ago enrolled him in the life-saving international study of the drug siltuximab, the first-ever drug for Castleman disease approved in 2014 by the Food and Drug Administration and the European Medicines Agency.
“I lucked out,” Guenther said.
Siltuximab is a major advance in the treatment of multicentric Castleman disease, an aggressive form of the disease that kills about 38 percent of patients within five years. With the new drug, the percentage of survivors should increase based on clinical trial results showing siltuximab successfully treated 34 percent of patients with multicentric Castleman disease.
Still, with the disease affecting an estimated 4,000 to 6,000 people in the U.S. alone, that leaves many without effective treatment. The drug is also not a cure, so patients like Guenther will have to take siltuximab the rest of their lives to avoid relapse.
Second Act
It took international collaboration among physician researchers and their patients to get siltuximab to market. That same kind of collaboration is being used in hopes of continued groundbreaking discoveries and other advances such as standard diagnostic criteria, van Rhee said.
Van Rhee is a founding member of the Castleman Disease Collaborative Network (CDCN), a worldwide community of physicians, researchers, patients and supporters working to combat the disease. Research and patient support are core missions for the CDCN, linking people through its website, www.cdcn.org. The website’s components include patient information, such as where to find medical treatment, and the latest information about the disease. The group is also developing diagnostic criteria for physicians to help remove the element of luck.
“You have a disease that is fairly complex and it’s very rare, so that’s a set up for failure to some extent isn’t it?” van Rhee said. “That’s why it’s important to get some solid information out there. “
The CDCN is developing a patient registry and biorepository of tissue and blood samples to aid with its research component. Much of the organization of the collaborative and its website development has been led by one of van Rhee’s patients, David Fajgenbaum, M.D., M.B.A. His inspiring success story while battling Castleman disease was recently featured in the Philly Voice.
A major barrier to finding and tracking Castleman disease patients is the lack of ICD 9 codes for the disease. Used primarily for billing purposes, such international codes are entered into medical records and stored in databases, giving researchers the ability to query them and to track how many people have certain diseases. The CDCN is pursuing ICD 9 codes for three major forms of Castleman disease: Multicentric Castleman disease; idiopathic (unknown cause) multicentric Castleman disease, and Unicentric Castleman disease, a form in which lymph nodes are enlarged in one area and can usually be cured with surgery.
The CDCN also raises money for research. One of its funded studies is being led by van Rhee, who hopes to identify a specific “fingerprint” for Castleman disease by analyzing the blood proteins of patients.
“Although Castleman disease is suggested to always be driven by Interleukin 6 (a protein involved in inflammation and infection responses), there are some patients who get sick without developing high Interleukin 6 levels,” van Rhee said. “So we want to have a better understanding of the biology of the patients.”
TRI Announces 2015 KL2 Award Recipients!
UAMS’ Bryce Marquis, Ph.D., an assistant professor of geriatrics, and Shona Ray-Griffith, M.D., an assistant professor of psychiatry, were recently named recipients of the Translational Research Institute’s 2015 KL2 Mentored Research Career Development Awards.
Marquis’ KL2 project is testing nutritional therapies to improve respiratory efficiency for heart failure patients. He anticipates the results of his work will direct the development of a new nutritional approach that can be used alone or with exercise to improve health outcomes in heart failure patients.
Ray-Griffith’s KL2 project is the first study using repetitive transcranial magnetic stimulation (rTMS) to treat neuropathic pain in pregnant women. rTMS uses a magnetic force to change the way nerves work in the brain. Because it is non-invasive and localized, rTMS is attractive for use in special populations, such as pregnancy, said Ray-Griffith, who has a secondary appointment in the Department of Obstetrics & Gynecology.
For the next two years, the KL2 awards will provide Marquis and Ray-Griffith with 75 percent of their salaries (up to $95,000), and up to $25,000 for research, tuition, travel expenses and education materials in support of their career development plans.
Marquis’ mentors are Robert Wolfe, Ph.D., Gohar Azhar, M.D., and Jeanne Wei, M.D., Ph.D., all in the Department of Geriatrics; Elisabet Borsheim, Ph.D., in the Department of Pediatrics; and Gunnar Boysen, Ph.D., in the College of Public Health. Marquis joined the UAMS College of Medicine faculty this year from the University of Central Arkansas, where he was an assistant professor of chemistry. He received his doctorate in analytical chemistry at the University of Minnesota, Minneapolis. He was also a National Research Council postdoctoral associate at the National Institute of Standards and Technology, Gaithersburg, Md.
Ray-Griffith’s mentors are Pedro Delgado, M.D., and Zachary Stowe, M.D., both in the Department of Psychiatry; and Everett Magann, M.D., in the Department of Obstetrics & Gynecology. She joined the UAMS College of Medicine faculty in 2013 with clinical appointments in the Women’s Mental Health program and as a psychiatry consult and liaison. She received her academic appointments in 2014 in the departments of Psychiatry and Obstetrics & Gynecology. She was a research fellow in the Women’s Mental Health Program and also served her residency and internship with the Department of Psychiatry. Ray-Griffith received her medical degree from the University of Texas Medical Branch in Galveston, and she is certified by the American Board of Psychiatry and Neurology.
NCATS Seeks Applications to Repurpose Existing Drugs
The National Center for Advancing Translational Sciences (NCATS) is seeking applications for rigorous, pre-clinical research projects that are based on repurposing existing drugs or biologics. Through this new funding opportunity, NCATS anticipates committing $4.3 million in fiscal year 2016 to issue 10 to 15 awards in support of studies that establish the rationale for a clinical trial.
Pre-clinical studies funded through this initiative will serve as “use cases” to demonstrate the utility of an independent crowdsourcing effort or of a computational algorithm to predict new therapeutic uses of an existing drug or biologic. The goal of an individual project must be to explore the potential new use of an existing investigational therapeutic or one already approved by the Food and Drug Administration to treat another disease.
Interested researchers should submit a letter of intent by Dec. 13, 2015. Applications are due Jan. 13, 2016.
To learn more about RFA-TR-16-001, contact Therapeutics.Discovery@nih.gov.
TRI Offering Research Forums
Nov. 30, 2015 | Do you have a research idea and need some assistance moving forward? Have you submitted a grant application that did not get funded? The Translational Research Institute (TRI) may be able to help through its Research Forums. Research Forums are individually tailored help sessions to address your specific needs. They are private meetings between you and a panel of experts that can help you move your research ideas and projects forward.
Over the past year, TRI has hosted 10 Research Forums for researchers from UAMS, Arkansas Children’s Hospital, Central Arkansas Veterans Healthcare System, and the UAMS Northwest Campus. You can request a Research Forum at TRIServices@uams.edu.
Read here about the Research Forum experience of UAMS’ Aliza Brown, Ph.D.
Dec. 8 Seminar: ‘Introduction to Methods of Financing a Startup’
The next monthly Health Sciences Innovation and Entrepreneurship (HSIE) seminar will be Dec. 8 at 4 p.m., Cancer Institute, 10th floor, Walton Auditorium. The seminar, “Introduction to Methods of Financing a Startup,” will be presented by two Innovate Arkansas advisors: Ted Dickey, a chartered financial analyst and general partner of CDFP Capital’s real estate fund; and Mike Smith Jr., a managing member of Gravity Arkansas, a member-managed angel fund.
Sponsored by the UAMS Translational Research Institute and UAMS BioVentures, the seminar series is open to all. It is available via WebEx. Get details.
Meeting number: 808 443 283
Join by phone: +1-855-749-4750 U.S. Toll Free
+1-415-655-0001 U.S. Toll
Access code: 808 443 283
Global call-in numbers | Toll-free calling restrictions
Add this meeting to your calendar.
Can’t join the meeting? Contact support.




