Welcome to Your SUPER-powered WP Engine Multisite Install. This is your first post. Edit or delete it, then start blogging!
Uncategorized
Researchers Urged to Complete GCP Training

The NIH Policy on Good Clinical Practice (GCP) Training became effective January 1, 2017. This policy applies to NIH-funded investigators and site staff who are responsible for the conduct, management and oversight of NIH-funded clinical trials. An NIH Clinical Trial is defined as Research studies in which one or more human subjects are prospectively assigned to one or more interventions to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes. An intervention is defined as a manipulation of the subject or subject’s environment for the purpose of modifying one or more health-related biomedical or behavioral processes and/or endpoints.
If you are conducting an NIH-funded clinical trial, you will need to complete your training as soon as possible. There are several ways to do this:
- Complete GCP training using the CITI program at https://www.citiprogram.org/. Log in using the user ID and password that you used for your human subjects protection training, scroll down past the list of courses you have completed and select Add a Course. Select Good Clinical Practice (GCP) Basic Course.
- Complete NIH-developed GCP training. Send completion certificate to Catrice Banks-Johnson in the Office of Research Compliance (CRBanksjohnson@uams.edu).
- For social and behavioral researchers, you can log onto Blackboard and complete the NCATS-developed GCP training. View documents for the GoSignMeUp registration process and basic navigation functions for the Blackboard Course. A Certification of Completion will be generated upon the conclusion of the final module. Send completion certificate to Catrice Banks-Johnson in the Office of Research Compliance (CRBanksjohnson@uams.edu).
- Send record/certificate of current GCP training (i.e., from industry-sponsored trial participation, from ACRP or SoCRA certification, or from completion of UAMS Graduate School courses PHSC6043 or PGSP6101) to Catrice Banks-Johnson (CRBanksjohnson@uams.edu).
For options 2-4 above, Catrice will record this training in Training Tracker for you upon receipt of your completion certificate.
NOTE: GCP training expires after three years.
If you have ambitions of conducting an NIH-funded clinical trial, you are highly encouraged to complete the training now!
Even if you have determined this does not apply to you, you are highly encouraged to complete the training now! GCP training is likely to be mandated within the next 6-12 months, as many institutions, journals, and other funding sources are trending toward this requirement.
The UAMS Office of Research Compliance will conduct random audits to ensure compliance with this policy.
If you have any questions, please contact one of the following institutional offices:
Amy Jo Jenkins
TRI
686-5939
ajjenkins@uams.edu
Jennifer Holland
IRB
526-7559
jrholland@uams.edu
Darri Scalzo
Office of Research Compliance
686-8062
dlscalzo@uams.edu
Larry Cornett, Ph.D.
Office of the Vice Chancellor for Research
686-5347
cornettlawrencee@uams.edu
TRI Research and Career Development Seminar Slides Available
Slides are now available from Susan Steelman, MLIS, UAMS head of education and reference services, who was the Feb. 22 speaker for the TRI Research and Career Development Seminar Series.
Download: Library 301: Publishing Pitfalls & Resources for Researchers/Authors

The TRIbune Is Here!
The January-February TRIbune newsletter is here! This issue features some exciting developments in Big Data accessibility for UAMS researchers. We now offer researchers a new cohort tool and access to a larger data network. You’ll also read about Amhad Baghal, M.D., our first director of the Arkansas Clinical Data Repository (AR-CDR), formerly the UAMS Enterprise Data Warehouse. Baghal, who arrived in October, is continuing to build on the new opportunities now available, so stay tuned! TRI’s Beatrice Boateng, Ph.D., director of evaluation and continuous improvement, is the subject of our TRI & Me feature, and we include your TRI-cited publications.
Cancer Institute’s Kacie Simpson Honored for Work with Research Participants

Kacie L. Simpson, the clinical research associate team lead at the UAMS Winthrop P. Rockefeller Cancer Institute, is the 2016 Bonny Hope Wallace Award recipient for her outstanding work with research participants.
The award was presented by Sandy Annis, a past recipient of the award who directs the UAMS Cancer Clinical Trials Office, at a Jan. 27 ceremony. Simpson was chosen for the award by members of the UAMS Certified Research Specialist Program.
Simpson has been at the Cancer Institute for 10 years. For the last nine years she has worked with patients in cancer clinical trials. She has served many roles in the Cancer Clinical Trials and Regulatory Affairs offices, including regulatory specialist, study coordinator, and for the last four years, manager of the study coordinators.
“It was an honor to be nominated for the Bonny Hope Wallace Award and selected by my peers to receive the award,” Simpson said. “I am also honored to work with world class physicians, nurses, research staff, and most importantly, the people who participate in research at the Cancer Institute.”
Simpson is a member of the Society of Clinical Research Associates (SoCRA), Association of American Cancer Institutes (AACI), the SWOG Oncology Research Professional (ORP) Liaison Committee, and the study coordinator for the SWOG Melanoma Committee. She has had UAMS Certified Research Specialist (CRS) certification since 2008 and SoCRA Certified Clinical Research Professional (CCRP) certification since 2010.
Wallace is remembered for her respectful treatment of research participants and her commitment to research integrity. She worked in research at UAMS for more than 30 years before her death in 2004.
Recipients of the award in Wallace’s name must demonstrate dedication to the research participant; respect for the participant’s sacrifice; devotion to research integrity; commitment to mentoring; and enthusiasm for learning.
Wallace was an instructor in surgery and laboratory director for surgical research at the Department of Surgery at UAMS as well as clinical coordinator of research at the Arkansas Children’s Hospital Burn Unit. Her efforts were focused on cutting-edge research to promote women’s health. Her accomplishments were many and her awards of recognition are numerous.
UAMS’ Data Sluice Machine
If new biomedical discoveries are like gold, to borrow the metaphor used by Meredith Zozus, Ph.D., the prospecting days are fast coming to an end. A new associate professor in the rapidly expanding Department of Biomedical Informatics (DBMI), Zozus was explaining UAMS’ recent and forthcoming biomedical informatics strategies to benefit clinicians, biomedical researchers and graduate students.
The need for managing enormous amounts of data and meeting the NIH’s new, higher expectations for rigor and study reproducibility are helping drive UAMS’ efforts for more robust data management systems. Another factor is the increasing difficulty compared to 20 years ago for a clinical researcher to make a single discovery that improves health outcomes, Zozus said.
“Back then, finding those gold nuggets was a lot easier. You could do a large study and learn something new that changed clinical practice and improved outcomes,” she said. “But those gold nuggets have become harder to find. So, like real-life prospectors, we’re moving from panning for gold to computationally sifting through tons of data to find the nuggets.”
Zozus also noted that the NIH, through its Big Data to Knowledge (BD2K) initiative, is targeting a growing shortage in biomedical research of individuals with computational expertise, informatics and statistics, with enough understanding of the underlying biology, biochemistry or physiology to really collaborate with a biomedical scientist. The challenge is particularly acute for extremely large datasets, where different methods are needed.
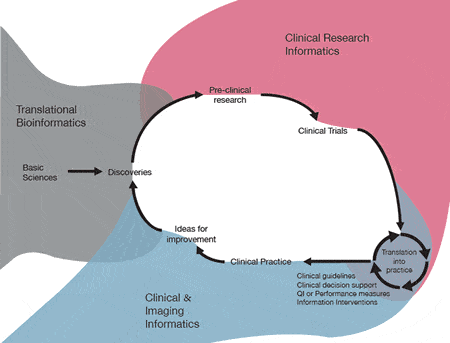
To help ensure UAMS is a leader in biomedical informatics, Fred Prior, Ph.D., who chairs DBMI and leads TRI’s Comprehensive Informatics Resource Center, recently announced new – some nationally unique – education initiatives to the TRI Leadership Council. Together the initiatives cover the translational spectrum, from molecules to populations. Pending approval from the Arkansas Department of Higher Education (ADHE), and starting in fall 2017, these four tracks in different areas of Biomedical Informatics will offer certificates, master’s degrees and doctorates:
Translational Bioinformatics. This degree program is for researchers using data in cellular and molecular level studies that have a clinical target, e.g., a study of genes producing a protein that has a role in disease. The field also encompasses work with pre-clinical data as development and testing begins for new targets, compounds or devices.
Imaging Informatics. Training in Imaging Informatics, offered only a few places nationally, includes coursework in research imaging to teach the latest modalities and methods of managing and interpreting images used in research. It also includes coursework for imaging professionals, i.e., those who run a hospital’s picture, archiving and communication (PAC) system where images such as CT scans, X-rays, and MRIs are stored.
Clinical Informatics. This program is for those interested in generation, management and use of information in health care settings, i.e., clinical decision support or algorithms to predict patients who are likely to respond well to treatment. Clinical informatics is a medical subspecialty approved in 2011 by the American Board of Medical Specialties. DBMI ran its first review course this summer to help physicians sitting for the clinical informatics board exam. The review class will be offered again this summer as a free service to all physicians in Arkansas who want to sit for the exam. Other plans include a fellowship in clinical informatics.
Clinical Research Informatics. If approved by ADHE, UAMS may be the first program to offer graduate degrees in Clinical Research Informatics in the United States.
Clinical research informatics involves data for the design, conduct and reporting of clinical studies. While easily confused with clinical informatics, clinical research informatics is not research on clinical informatics; instead, it is the informatics of research, i.e., how information is used in gauging the feasibility, or in designing, conducting or reporting clinical studies. If approved by ADHE this spring, the Clinical Research Informatics track will include a master’s degree and Ph.D. as well as a professional master’s option.
All four tracks will have the option of distance learning.
DBMI and TRI will also continue the Research & Application Seminar Series, which is open to the public and offers an hour of CME credit to clinical and informatics professionals across the state for attending seminars on the latest biomedical research tools and practices.
While biomedical informatics is not new to UAMS, it is increasingly approaching the data as a science, considering its fundamental properties and how those should govern its management, Zozus said.
“As an institution we are increasingly providing collaboration, informatics expertise and data infrastructure to biomedical researchers at all scales, from the smallest to the largest of studies, so our investigators don’t have to build their own data processes,” she said. “In essence, we are building a data sluice operation.
UAMS Graduates 29 Certified Research Specialists
Twenty-nine UAMS research professionals received Certified Research Specialist (CRS) certificates at a graduation ceremony on Jan. 27. The graduation followed training that occurred in 2016.
The CRS program, managed by the Office of Research Compliance, requires that participants maintain Collaborative Institutional Training Initiative (CITI) Human Subjects Research training, complete 28 hours of relevant coursework, and pass a comprehensive proficiency exam. Graduates of the program earn the “CRS” credentials and maintain their certification status by completing six contact hours in applicable coursework in subsequent years.
The Translational Research Institute (TRI) requires its research staff to complete and maintain CRS certification, and other UAMS departments have also adopted CRS certification as a job requirement for certain research-related positions.
The 2016 Certified Research Specialist Graduates are:
- Suzan Blair, College of Medicine (COM), Dermatology
- Ashley Block, COM PEDS Birth Defects
- Amanda Bull, COM Geriatrics
- Pamela Christie, TRI Administration
- Makethia Curtis, COM, Family and Preventive Medicine
- Giuseppina Dusio, COM, Nephrology
- Ashley Funderburg, College of Nursing, Geriatrics
- Patricia Gminski , COM, Pathology
- Susan Goolsby, Arkansas Children’s Hospital (ACH), Clinical Nutrition
- Paul Griffiths, Myeloma Institute
- Kathleen Hicks, COM, Pulmonary
- Holly Jarrell, Myeloma Institute
- Susannah Kirby, TRI Project Support Unit
- Matthew Kovak , Cancer Clinical Trials
- Teri Landrum, Nursing Services
- Austin Lovenstein, ACH Nursing Research
- Karen Mack, Nursing Services
- Lisa McIntos, Office of Sponsored Programs Administrative Network
- Heather Moody, OB/GYN Research
- Veronica Overton, Psychiatric Research Institute
Entrepreneurship Series Begins Today, Expands to Other CTSA Institutions

The 2017 Health Sciences Entrepreneurship Seminar Series begins Feb. 1, with UAMS’ Amy Hester, Ph.D., R.N., director of Nursing Research and Innovation, speaking from 5 – 6 p.m. at the Reynolds Institute on Aging, Jo Ellen Ford Auditorium.
Hester will present “Innovating in Healthcare: Idea Formation to Revenue Generation and Everything in Between.”
Hester’s research focuses on falls and injury prediction and prevention across the continuum of care. She is the inventor of multiple products and co-founded the biotechnology company HD Nursing, LLC in 2012, for which she is chief scientific officer.
The UAMS Seminar Series this year is being offered in collaboration with the University of Alabama, Birmingham, University of Kansas Medical Center and the University of Utah – all Clinical and Translational Science Award (CTSA) institutions. The series is sponsored by the NIGMS Systems Pharmacology and Toxicology T32 Training Program, UAMS Translational Research Institute (TRI) and UAMS BioVentures.
Congratulations to Our Yeti Winners!

UAMS’ Youzhong Yuan, M.D., Ph.D., in the Department of Pathology, was our third and final Yeti Tumbler winner in drawings to celebrate UAMS Profiles’ recent system upgrade and first anniversary.
Kandi Stallings-Archer, M.D., won a Yeti Tumbler in our second drawing, and Jonathan Goree, M.D., was our first winner.
Profiles is a networking tool designed to help researchers, clinicians, educators and other faculty members connect with one another through their research/clinical/academic interests. All faculty members have a Profiles page, and all are encouraged to visit the site and edit/add to their personal profile.
Research Highlighted in 2016 UAMS Achievements

UAMS saw numerous achievements in 2016, including in research. The year was highlighted by a $41.8 million NIH award to oversee a 17-site pediatric clinical trial network that will provide medically underserved and rural children access to clinical studies on the effect of environmental influences on early development.
Read more: http://bit.ly/2hKc71W

