The Arkansas Clinical Data Repository (AR-CDR) is a research data warehouse that provides a single and secure source of data for use in clinical and translational research. Since its launch in 2011, the AR-CDR has enabled UAMS researchers and faculty to conduct high quality, timely and efficient clinical and translational research. The AR-CDR houses data that are extracted from EPIC electronic health records (EHR) system and legacy systems. Types of data in the AR-CDR include:
Patient Data
- Demographics
- Admissions
- Visits
- Inpatient/Outpatient
- Providers
Clinical Data
- Diagnoses
- Immunizations
- Laboratory Results
- Procedures
With the help of emerging technologies, UAMS continues to enhance the AR-CDR with technical capabilities that:
- Empower researchers through self-service access and ability to ask disease-specific research questions.
- Integrate clinical and genomic data that harness Big Data to offer personalized treatments.
- Leverage natural language processing (NLP) technology to extract knowledge from unstructured data.
Getting Started with a Research Study
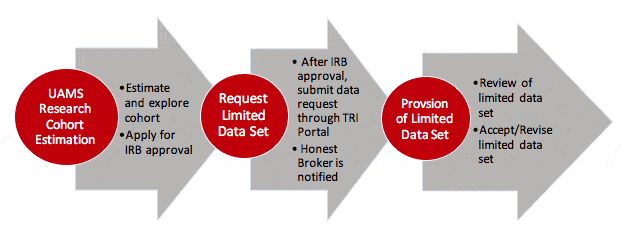
To gain access to the UAMS Research Cohort Estimation Tool, you will need to:
- Complete the brief (10 minute) online training module.
- Submit a request for AR-CDR access through the CIRC Request Services Portal.
- Access to the UAMS Research Cohort Estimation tool allows investigators to query AR-CDR to obtain aggregate data (patient counts) according to patient population characteristics.
To request identifiable patient data, you will need to:
- Submit a request to the IRB for approval.
- Once IRB approval is obtained, submit a request for a limited data set through the CIRC Request Services Portal.
AR-CDR will work with the Honest Broker to coordinate and review the data requirements of the limited data request and then deliver the requested data to the investigator in a timely manner.
